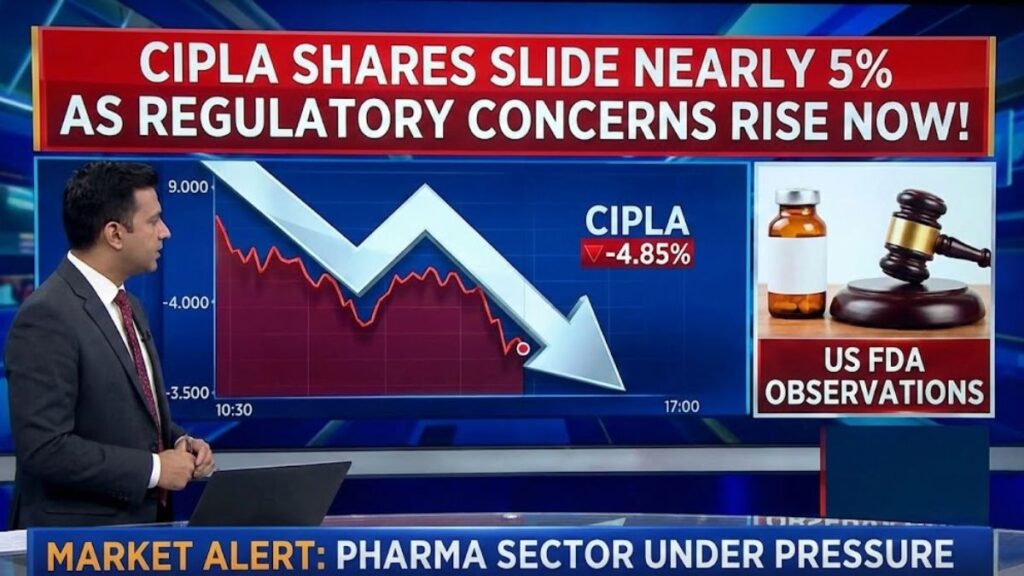
Indian pharmaceutical major Cipla Ltd. saw a sharp decline in its share price on Wednesday, January 7, with the stock falling as much as 4.9 percent intraday amid fresh regulatory concerns and broader market weakness. Cipla emerged as one of the biggest losers on the NIFTY50 index, underperforming both the sector and key benchmark indices.
Stock Movement and Market Context
On the National Stock Exchange (NSE), Cipla’s stock dropped to around ₹1,455 per share at its intraday low, down nearly 5 percent from the previous session, as selling pressure intensified. The company also finished the session as one of the top decliners among major listed stocks, slipping over 4 percent to ₹1,465 by the close.
The broader market showed mixed signals on Wednesday. While key indices like the SENSEX and NIFTY50 ended slightly lower for the third straight session, sectoral weakness — particularly in auto and oil & gas — compounded losses in heavyweight counters like Cipla.
Regulatory Concerns Drive Selling Pressure
Investor sentiment was dampened primarily by reports related to regulatory observations from the United States Food and Drug Administration (USFDA) at a contract manufacturing partner’s facility connected to Cipla’s plans for the US market.
The USFDA highlighted several compliance gaps, including weaknesses in contamination‑control procedures and aseptic processing systems at the Greece‑based plant run by Pharmathen International, with which Cipla has a partnership to commercialise the cancer drug lanreotide.
While such regulatory Form 483 observations do not automatically halt production, they can delay approvals or affect launch timelines for key products in the lucrative U.S. market, which is a significant revenue driver for Indian pharma exporters. Market participants often react to these observations as potential near‑term risks until the company and its partner address the findings and receive follow‑up confirmation from regulators.
Technical and Sector Dynamics
Analysts tracking the stock noted that Cipla’s technical indicators appeared weak, with the share trading below all key moving average levels — a sign of bearish momentum in the short and long term. This technical weakness, combined with broader pharma sector pressures, contributed to the stock’s underperformance relative to its peers.
Additionally, option market data showed heavy put option activity, suggesting a pronounced bearish sentiment or increased hedging by traders anticipating further near‑term downside or volatility in the stock.
In contrast, other sectors such as midcaps and smallcaps exhibited resilience on Wednesday, highlighting that Cipla’s decline was influenced more by stock‑specific developments than broad market weakness.
Company Developments and Growth Prospects
Despite the share price weakness on January 7, Cipla has been active on the product front. In December 2025, the company partnered with Stempeutics Research to launch Ciplostem, a new therapy approved by India’s Drug Controller General (DCGI) for treating knee osteoarthritis, a major degenerative joint condition. This move marked Cipla’s foray into orthobiologic medicine, offering a disease‑modifying treatment for patients with Grade II and III knee osteoarthritis.
Cipla also recently launched Yurpeak (tirzepatide), a weekly injectable therapy for obesity and type‑2 diabetes mellitus, capitalising on rising demand for metabolic disease treatments in India. The drug represents the second branded version of tirzepatide that Cipla will distribute in the country after receiving DCGI approval.
These product introductions reflect Cipla’s efforts to diversify its portfolio and capture growth in both specialized therapies and chronic care segments, even as near‑term regulatory developments create volatility in its stock performance.
Broader Sector Outlook
Pharmaceutical stocks often react sensitively to regulatory news, especially concerning the U.S. FDA — a key regulator and export market for Indian drugmakers. Past episodes have shown that observations and compliance issues can affect investor sentiment until resolution or re‑inspection clarifies the company’s path forward.
Motilal Oswal and other brokerages have maintained cautious views on Cipla in the past due to delayed niche product approvals and slower earnings expansion compared with some peers, reflecting limited near‑term upside despite strong fundamentals.
What Investors Should Watch Next
Investors and analysts will likely monitor the following developments closely:
- Regulatory updates from the U.S. FDA or Pharmathen’s corrective action plans, which could affect Cipla’s launch timeline for Lanreotide and other products tied to U.S. sales.
- Upcoming earnings results, including guidance on margins, export performance, and growth in newly launched therapies like Yurpeak and Ciplostem.
- Sector and index trends, as broader market conditions and foreign institutional investor activity continue to influence heavyweight stocks such as Cipla.
Conclusion
Cipla’s share price decline of nearly 5 percent on January 7 underscored the sensitivity of pharma stocks to regulatory and technical headwinds, particularly when linked to the U.S. market and contract manufacturing partners.
While the company continues to expand its product lineup and pursue growth in key therapeutic areas, near‑term volatility driven by compliance concerns and market positioning will be key factors for investors to monitor in the weeks ahead.